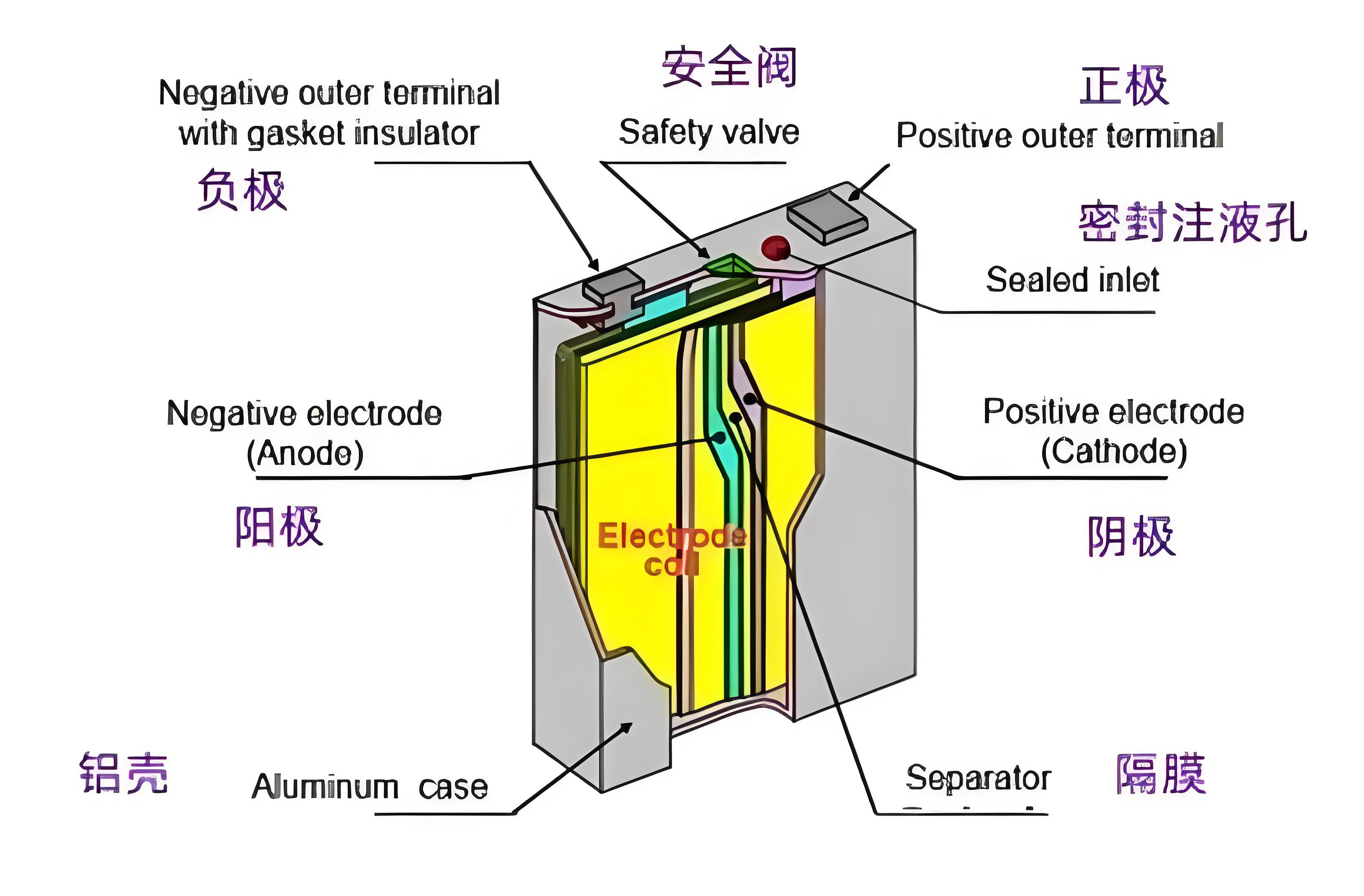
To understand how lithium-ion batteries work, you first need to understand their "four core members." A battery is primarily composed of a positive electrode, a negative electrode, an electrolyte, and a separator. These four work together to store and release energy.
The positive electrode is mostly made of lithium compounds, such as lithium iron phosphate (LFP), ternary materials (nickel cobalt manganese NCM), etc., which are the "starting point" of lithium ions; the negative electrode is usually carbon materials such as graphite, which is the "destination" of lithium ions and can efficiently adsorb lithium ions through its layered structure; the electrolyte is generally an organic solution dissolved with lithium salts, like a "highway", providing a channel for the movement of lithium ions; the diaphragm is the key "safety guard", which is a porous film that allows lithium ions to pass through but can prevent electrons from shuttling, avoiding direct contact between the positive and negative electrodes and causing short circuits.
Discharge process: lithium ions' "homecoming journey"
When a battery powers a device, the discharge process officially begins. Lithium ions in the positive electrode gain energy and escape from the crystalline lattice of the positive electrode material, moving towards the negative electrode via the electrolyte, a "highway" that acts as a "leave home." Meanwhile, electrons, unable to pass through the separator, flow from the positive electrode to the negative electrode through an external circuit, forming an electric current that powers devices like POS terminals, scanners, and sweepers. This is like electrons taking a detour to power a device.
When lithium ions reach the negative electrode, they embed themselves into the graphite's layered structure, like "coming home and settling in." At this point, the negative electrode is lithium-rich, while the positive electrode is lithium-poor. As discharge continues, the lithium ions in the positive electrode decrease, and the battery's charge gradually decreases. Until most of the lithium ions in the positive electrode have transferred to the negative electrode, the battery needs to be recharged.
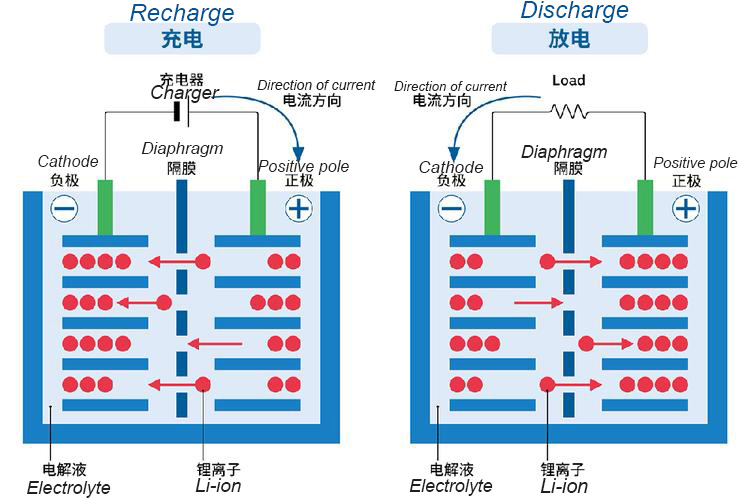
Charging process: the return journey for lithium ions
During charging, an external power source is connected to the battery, providing energy to the system. Under the influence of the electric field, lithium ions previously embedded in the graphite layer of the negative electrode are "ready to go," emerging from the negative electrode and moving through the electrolyte to the positive electrode, completing the "return trip." Simultaneously, electrons flow from the negative electrode to the positive electrode through the external circuit, where they recombined with lithium ions and became embedded in the positive electrode material's lattice, restoring the positive electrode to a lithium-rich state and the negative electrode to a lithium-poor state.
This process is like "refueling" the battery. As lithium ions continue to return from the negative electrode to the positive electrode and are stored, the battery's charge gradually recovers. It's worth noting that a high-quality battery management system precisely controls the charging current and voltage to avoid damage to the battery caused by overcharging or overfilling, thereby extending the battery's lifespan.
Principles supporting applications: from everyday devices to energy revolution
The working principle of lithium-ion batteries dictates their advantages, such as high energy density, long cycle life, and lack of memory effect. These advantages have enabled them to shine in numerous fields. In consumer electronics, devices such as POS terminals and laptops, thanks to their compact size and long battery life, meet people's daily needs. In the field of new energy vehicles, the high energy density of lithium-ion batteries can support vehicles for hundreds of kilometers, contributing to the automotive industry's transition to a green and low-carbon economy. In the field of energy storage, large-scale lithium-ion battery energy storage systems can store clean energy such as solar and wind power, mitigate energy supply fluctuations, and promote energy structure upgrades.
With continuous technological innovation, researchers are continuously improving the performance of lithium-ion batteries by optimizing positive and negative electrode materials, refining electrolyte formulations, and enhancing separator performance. For example, the high-density lithium iron phosphate cathode materials and solid-state battery technologies mentioned above are all based on in-depth research on the laws of lithium-ion motion. These breakthroughs have further overcome bottlenecks in battery energy density and safety, allowing lithium-ion batteries to play a greater role in the green energy revolution.
Conclusion: Tiny ions contain huge energy
Lithium-ion batteries may seem compact, but the orderly movement of lithium ions within them underpins the energy needs of numerous sectors of modern society. From everyday electronic devices to new energy vehicles and energy storage systems driving the global low-carbon transition, the working principles of lithium-ion batteries remain central to their efficient and safe operation. With continuous technological advancements, we believe lithium-ion batteries will continue to improve, injecting further momentum into the development of green energy worldwide.















