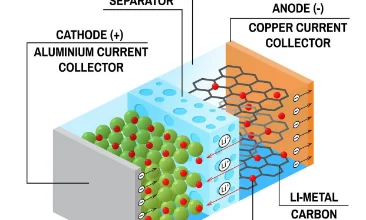
As consumer electronics products such as smartphones, wearable devices, and foldable screen computers are upgraded towards thinner, lighter designs with longer battery life and enhanced safety, the lithium-ion battery, as the core energy source, is undergoing a comprehensive transformation in its internal structure design and material selection. Recently, industry experts, through the disassembly and analysis of mainstream consumer electronics lithium-ion batteries, have found that the optimization of all component structures—from the positive and negative electrodes and electrolyte to the separator and casing—not only directly determines the battery's energy density, fast charging speed, and safety performance, but also serves as a key driving force for the iteration of the consumer electronics industry.
Positive Electrode: A multi-material composite design balances capacity and stability. Disassembly reveals that the positive electrode of consumer electronics lithium-ion batteries is the core carrier of energy storage, mainly composed of positive electrode active materials, conductive agents, binders, and current collectors. The selection and structural design of the active material directly affect the energy density. Currently, the mainstream positive electrode materials have formed a composite combination of "ternary materials + lithium iron phosphate": high-end flagship models mostly use high-nickel ternary materials (such as NCM811), increasing lithium-ion intercalation and deintercalation capacity by increasing the nickel content, enabling the cell energy density to exceed 300 Wh/kg; while mid-range models and wearable devices that prioritize safety and long cycle life prefer lithium iron phosphate materials, whose olivine structure is more stable, and the cycle life can reach more than 1500 cycles.

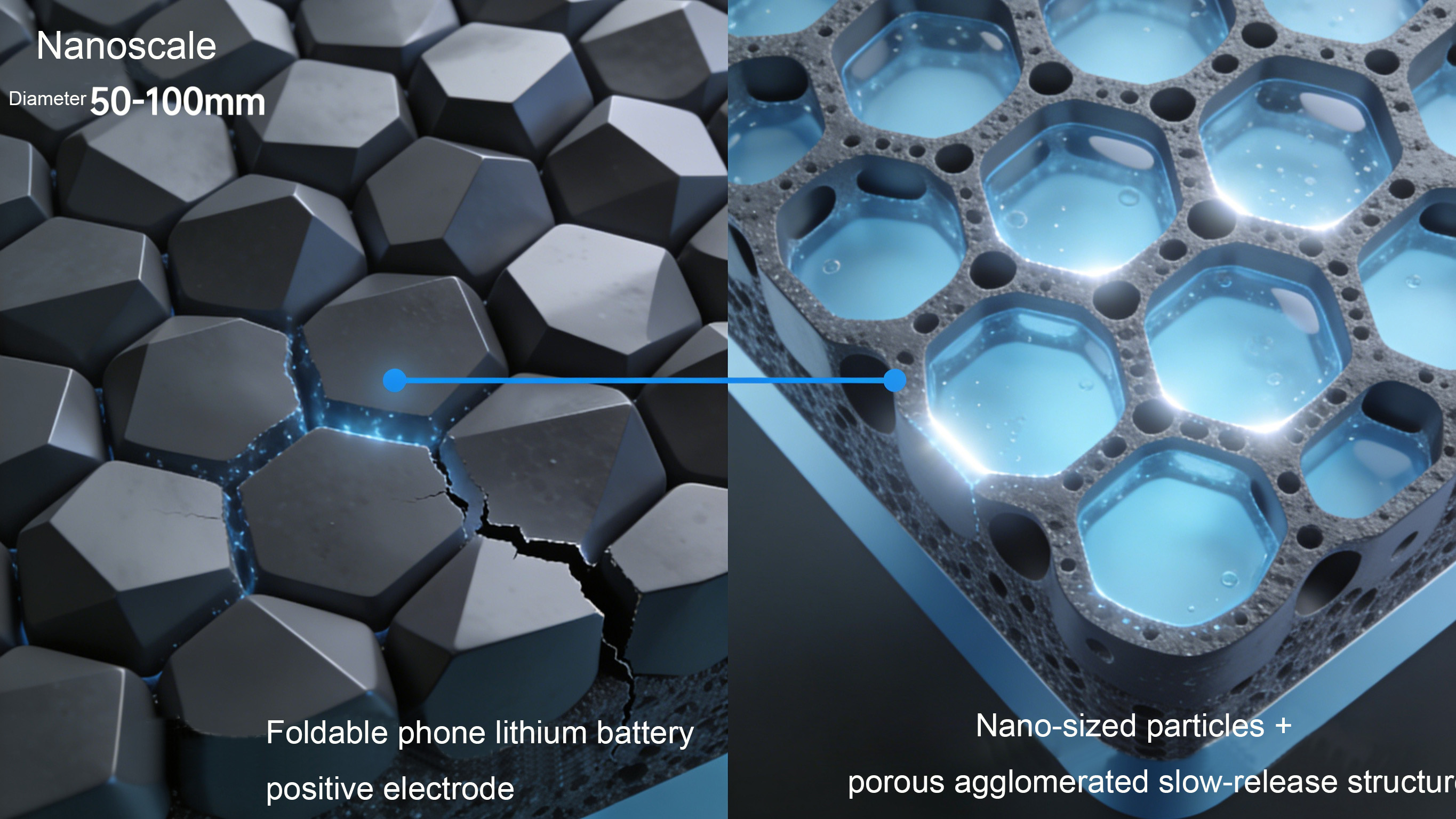
Notably, the optimization of the microstructure of cathode materials is becoming a key technological breakthrough. Analysis of the battery in the latest foldable screen smartphones reveals that its cathode utilizes a "nanoscale particle + porous aggregate structure" design. This design increases the contact area with the electrolyte, improving ion conductivity efficiency, and also mitigates volume expansion during charging and discharging, resulting in a more than 20% increase in battery cycle life. Furthermore, lithium manganese iron phosphate (LMFP), as a new type of cathode material, is gradually being applied to mid-to-high-end models. Through manganese doping to optimize the crystal structure, its energy density is 15-20% higher than traditional lithium iron phosphate, while retaining excellent thermal stability. It is currently being used in some models by well-known brands.
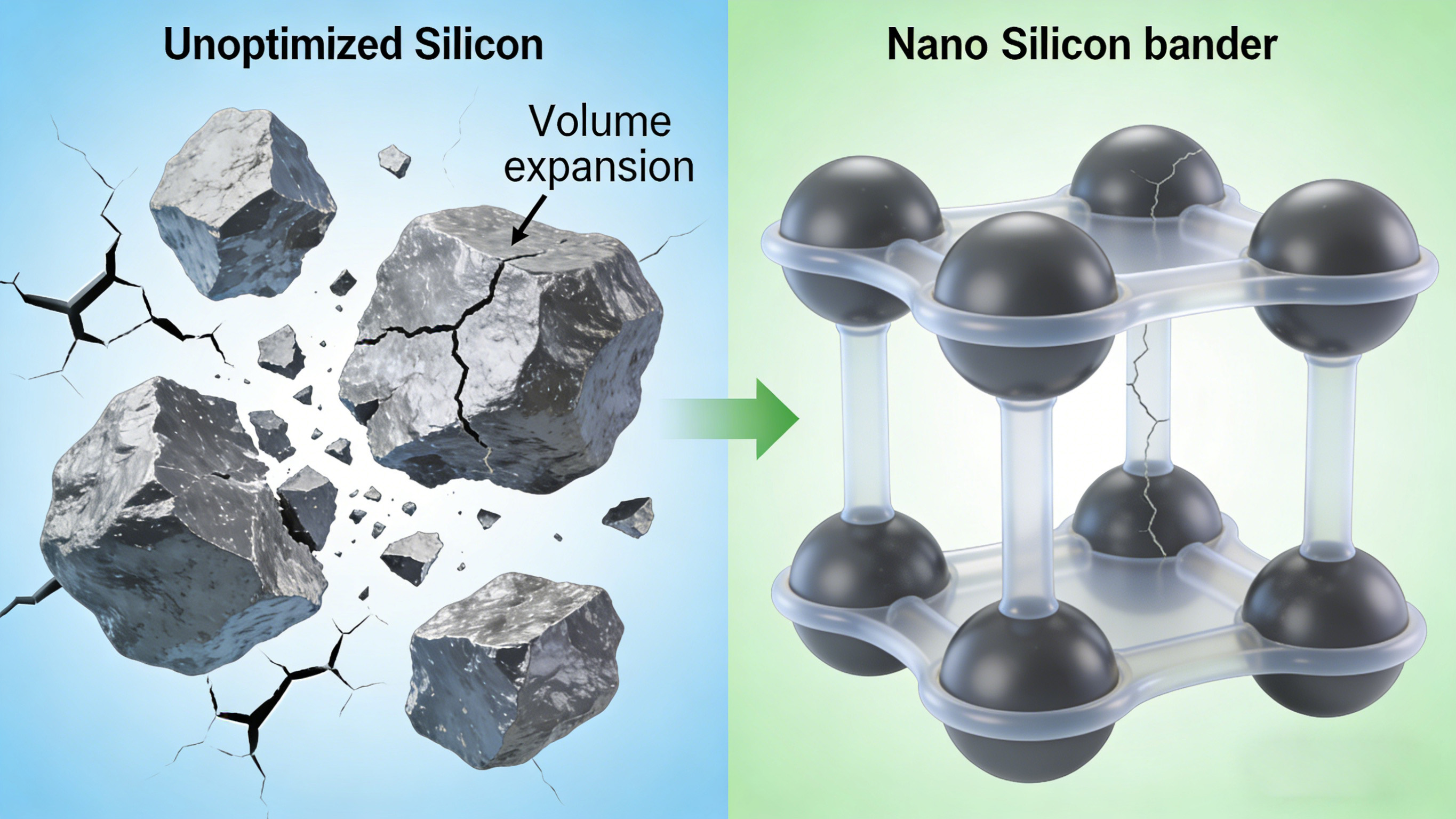
Negative Electrode: Scalable application of silicon-based materials breaks through capacity limitations. The negative electrode acts as a "temporary storage warehouse" for lithium ions. Traditional consumer electronics batteries primarily use graphite as the negative electrode material, but graphite has a low theoretical capacity (372 mAh/g), making it difficult to meet the long battery life requirements of high-end models. Disassembly of several high-end smartphone batteries reveals that silicon-based negative electrodes have become the mainstream configuration. By combining silicon material with graphite, a "silicon-carbon composite negative electrode" structure is formed, effectively overcoming capacity limitations. Silicon has a theoretical capacity of up to 4200 mAh/g, more than 10 times that of graphite. Adding 10-20% silicon content to the composite negative electrode can increase the energy density of the battery cell by 15-30%.

However, silicon materials undergo more than 300% volume expansion during charging and discharging, which can easily lead to electrode pulverization and detachment, affecting battery life. To address this challenge, the industry has adopted structural optimization techniques: firstly, using nano-silicon particles to replace traditional silicon powder to reduce structural damage caused by volume expansion; secondly, introducing elastic binders into silicon-carbon composite materials to form a "buffer layer" to alleviate expansion stress; and thirdly, forming a protective layer on the surface of silicon particles through carbon coating technology to improve material stability. Disassembly data shows that silicon-carbon composite anode batteries using these technologies can achieve a cycle life of over 1000 cycles, which is sufficient to meet the daily usage needs of consumer electronics.
Electrolyte and Separator: Upgrading the Invisible Defense Line, Strengthening the Safety Barrier. The electrolyte acts as the "bridge" for lithium-ion transport, and the separator acts as the "firewall" separating the positive and negative electrodes. Although neither directly stores energy, both are crucial for ensuring battery safety and performance. Disassembly reveals that mainstream consumer electronics batteries have widely adopted high-purity electrolytes and functionalized separators, forming a dual safety protection system. In terms of electrolytes, the addition of flame retardants, film-forming additives, and other functional components enhances the electrolyte's high-temperature resistance and oxidation resistance. The new electrolytes used in some high-end models remain stable even at 150°C, effectively suppressing the risk of thermal runaway.
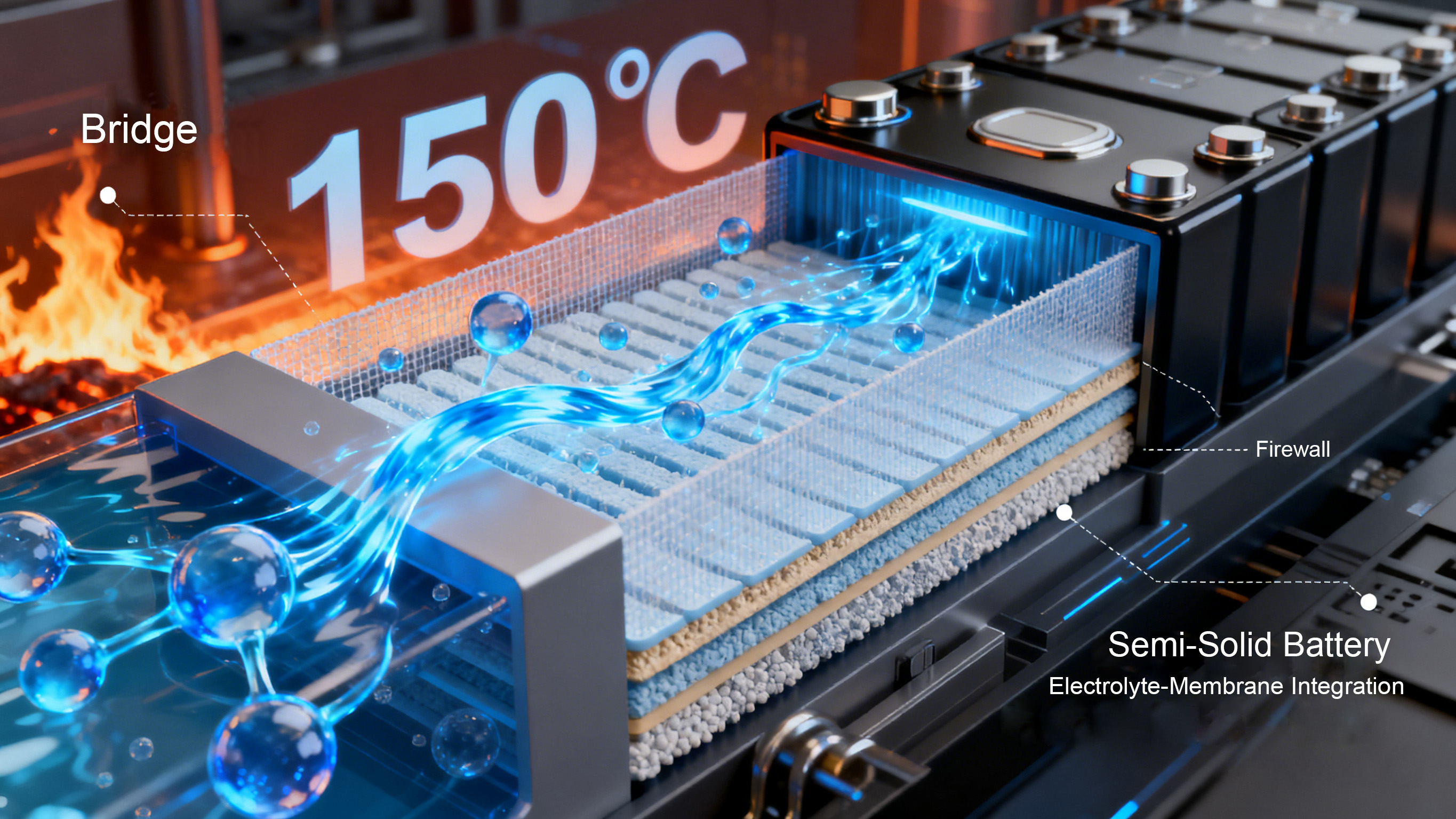
In the separator field, there is an upgrading trend from "single-layer to multi-layer, and from ordinary to functionalized." Traditional single-layer polyethylene (PE) separators have been gradually replaced by double-layer PE/PP composite separators. These composite separators possess excellent mechanical strength and thermal stability, and can rapidly shrink at high temperatures to create a circuit break, preventing short circuits between the positive and negative electrodes. More advanced ceramic-coated separators, achieved by coating the separator surface with ceramic materials such as alumina and boehmite, further enhance the separator's high-temperature resistance and puncture resistance. Disassembly tests show that batteries using ceramic-coated separators have a more than 50% increase in puncture resistance, significantly improving safety in extreme scenarios such as compression and drops. Furthermore, the gel electrolytes and solid electrolytes used in semi-solid-state batteries are gradually breaking down the boundaries between traditional electrolytes and separators. Through an "electrolyte-separator integrated" structure, they completely solve the leakage problem and have already achieved mass production in some foldable screen models.
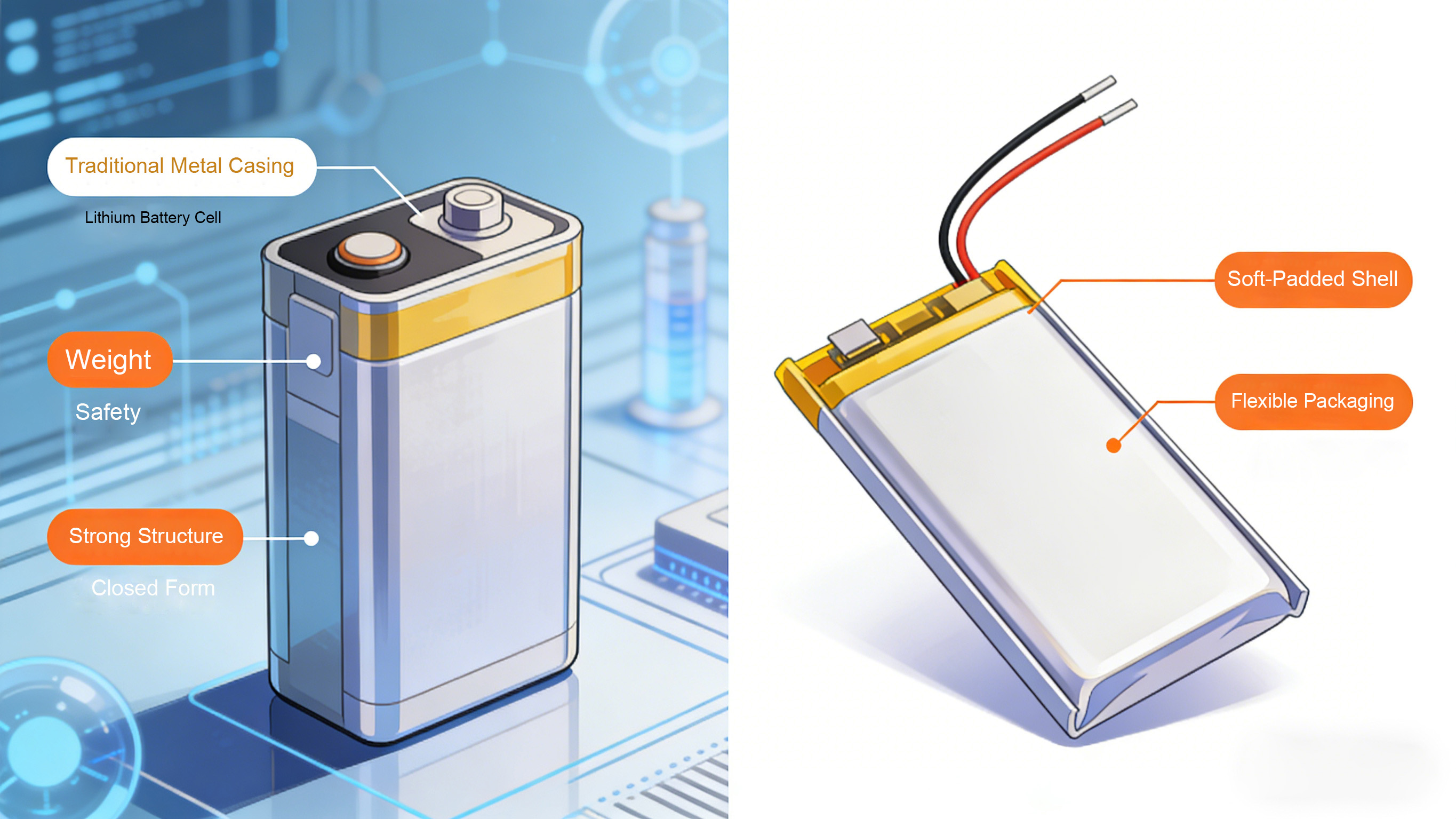
Casing and Current Collector: Balancing lightweight design with high strength to meet terminal design requirements. The pursuit of thinner and lighter consumer electronics devices is driving the upgrade of battery casings and current collectors towards "lightweight and thin" designs. Disassembly reveals that traditional metal casings have been gradually replaced by soft-pack casings. Soft-pack batteries use aluminum-plastic composite film as the casing, reducing the thickness by more than 30% compared to metal casings, and offering good flexibility to adapt to the design requirements of irregularly shaped terminals such as folding screens. However, there is also a trend of steel-cased batteries returning in high-end models. Some models use a sealed steel casing structure with a thickness of only 2.5mm, improving puncture resistance by 50% compared to soft-pack batteries, while also effectively enhancing the capacity stability of the battery cell.
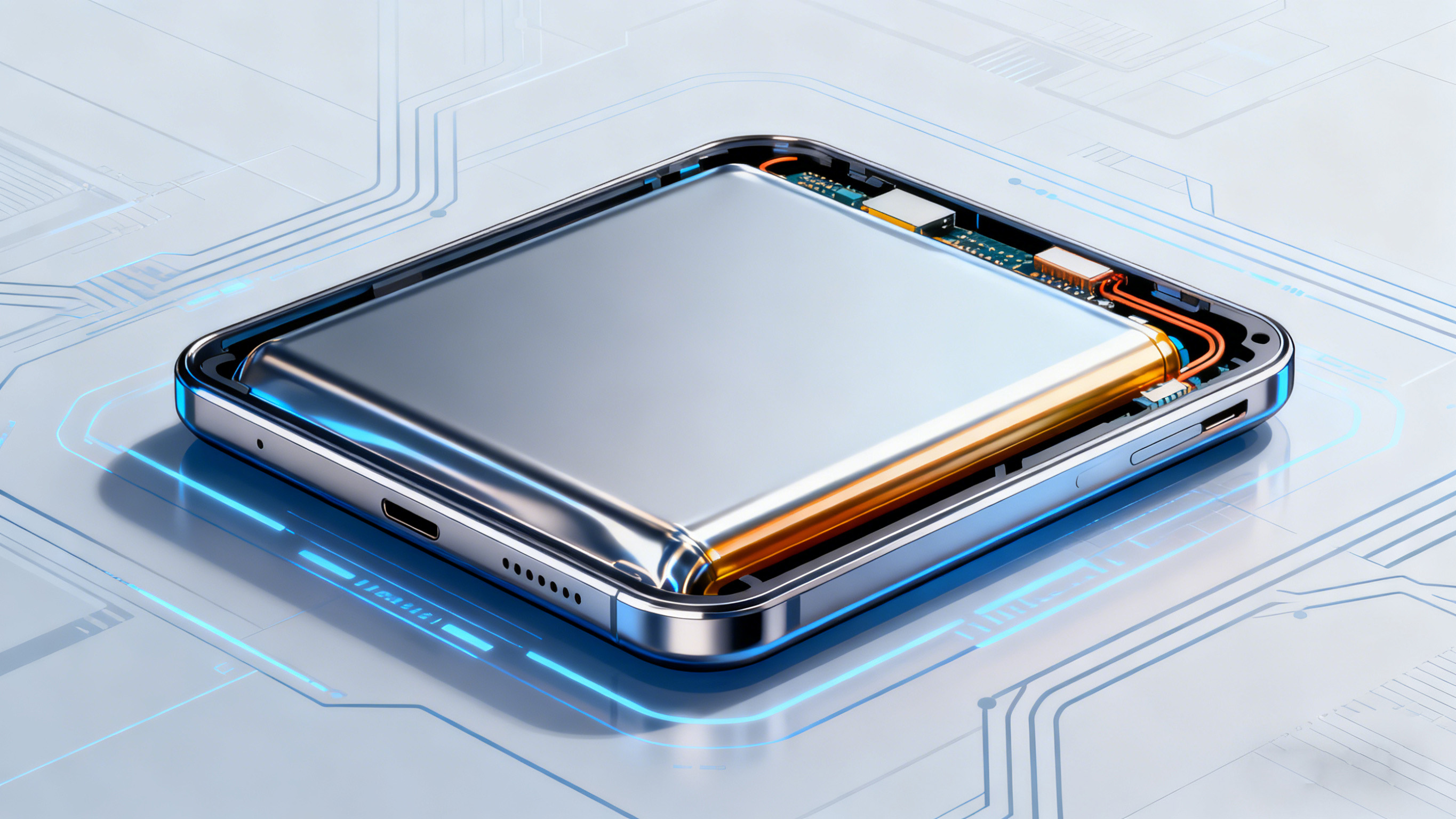
As the carrier for current transmission, the current collector is also undergoing lightweight optimization. Traditionally, aluminum foil is used for the positive electrode current collector and copper foil for the negative electrode. Currently, the industry is reducing the weight and volume of the current collector while maintaining conductivity by reducing the thickness of the foil material (from 12 μm to below 8 μm) and using porous structures. This frees up more space for active materials, further increasing the energy density of the battery cell. Disassembly data shows that batteries using ultra-thin current collectors can achieve a 5-8% increase in energy density, while reducing battery weight by more than 10%.
Industry experts say that the deconstruction of the internal structure of consumer electronics lithium-ion batteries clearly demonstrates the technological iteration path of "material innovation + structural optimization." In the future, with the advancement of semi-solid and all-solid-state technologies, the internal structure of batteries will undergo more profound changes, and the boundaries between the cathode, anode, electrolyte, and separator will gradually blur, forming an integrated energy storage unit. This transformation will not only drive breakthroughs in consumer electronics batteries in terms of "higher energy density, faster charging, longer lifespan, and improved safety," but will also provide core energy support for the implementation of emerging consumer electronics scenarios such as AR/VR and wearable medical devices.